 |

| Protein Science | ||
|
||
 |
||
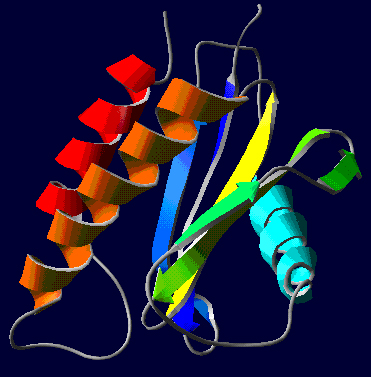
This is the structure of sedlin, why do mutations in this protein result specifically in skeletal disease?
|
||
The Structural Basis of Mutant Collagen X Protein Folding/Misfolding Collagen X is the most abundant extracellular matrix component and is synthesized specifically by hypertrophic chondrocytes. Mutations in Col10A1 result in Schmid metaphyseal chondrodysplasia (SCMD). We have recently shown in a mouse model of the disease that these mutations lead to the unfolded protein response and chondrocyte redifferentiation. We are currently probing the structural basis of mutations in collagen X and how these mutations effect correct protein folding and chain assembly.
|
||
 |
||
The C-terminal NC-1 domain of Collagen X is rich in beta sheet structures. Mutations in this protein cause SCMD.
|
||
Chondrocyte Proteomics: Delineating Protein Networks, Communication and Signaling Pathways Many skeletal disease result from protein misfolding, protein trafficking problems and other stresses occurring in chondrocytes. We are using Isotope Coded Affinity Tagging (ICAT) to perform comparative proteomics of chondrocytes and to elucidate the processes by which protein misfunction leads to disease. |