Academic Staff

Professor HERVAS MILLAN, Rubén
- BS (Autonomous U), PhD (Autonomous U & Cajal Institute)
- Assistant Professor
- Biophysics and protein structure
- Protein aggregation in physiological processes
- Structure and activity of functional amyloids
Ruben Hervas Millan is currently an Assistant Professor in the School of Biomedical Sciences at The University of Hong Kong. Ruben received his B.Sc. degree in Biochemistry from the Autonomous University of Madrid, Spain, and his Ph.D. in Molecular Sciences from the Cajal Institute & Autonomous University, after which he undertook postdoctoral training at the Stowers Institute for Medical Research in the United States of America. Following his postdoctoral training, Ruben joined The University of Hong Kong as an Assistant Professor in 2021.
The purpose of our research is to provide new structural and mechanistic information about specific protein assemblies that are linked with memory persistence and animal development.
Proteins are the workhorses in the cell. A given protein function is determined by its three-dimensional structure (fold/shape), which emerges from a complex folding process. Among these folds, amyloids are organized, filamentous protein aggregates commonly linked with pathological conditions, including Alzheimer’s or Parkinson’s disease. Nonetheless, amyloids also play a role in diverse physiological processes across multiple phyla. The knowledge of the principles that drive pathological protein aggregation is growing. However, how amyloids are functional units remains unclear. Partially, this is due to the absence of high-resolution structural data of functional amyloids.
Research in our lab focuses on the structure determination of functional amyloids using an electron microscope operating at cryogenic conditions; a technique called electron-cryo microscopy (cryo-EM). Recently, we have characterized the function and 3D structure of the aggregated state, isolated from the Drosophila brain, of the synaptic protein synthesis regulator CPEB, which across animal species plays a causal role in transforming a short-term memory into a long-lived one. Now, we aim to determine the structure and the biochemical activity of functional amyloids involved in both human memory persistence and animal development. Coupling cryo-EM with orthogonal data, such as in vitro activity tests or animal models to test the consequences of amyloid assembly and disruption, may provide insight into the functional consequences of amyloid formation in vivo and into how organisms regulate amyloid assembly/disassembly to restrict their activity in time and space. In addition, the derived findings may force us to reexamine why and how other amyloids are deleterious, particularly to the human nervous system, and how protein aggregation-based diseases, such as Alzheimer´s, might be treated in the future.
Our structures:
Here, we will summarize the main findings and structures derived from our research:
CPEB/Orb2 filaments from Drosophila brain (Hervas et al 2020, Science)
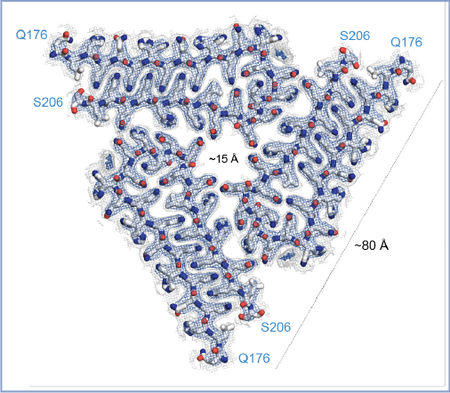
PDB: 6VPS, EMDB / ID: EMD-21316
We determined the atomic structure of the biochemically active, aggregated state of CPEB/Orb2 extracted from the Drosophila adult brain. Orb2 aggregates consist of elongated threefold-symmetric amyloid filaments stabilized through a tightly interdigitated glutamine packing. The hydrophilic nature of the Orb2 filament core suggests how the neuronal Orb2 amyloid could act as a stable yet malleable substrate of memory.
- Hervas R†,*, Fernández-Ramírez MC*, Galera-Prat A, Suzuki M, Nagai Y, Bruix M, Menéndez M, Laurents DV, Carrión-Vázquez M†. Divergent CPEB prion-like domains reveal different assembly mechanisms for a generic amyloid-like fold. BMC Biol 19(1):43. doi: 10.1186/s12915-021-00967-9 (2021)
- Hervas R†, Murzin AG, Si K†. Implications of the Orb2 amyloid structure in Huntington’s Disease. Int. J. Mol. Sci. 21(18), 6910. doi: 10.3390/ijms21186910 (2020)
- Hervas R, Rau MJ, Park Y, Zhang W, Murzin AG, Fitzpatrick JAJ, Scheres SHW, Si K. Cryo-EM structure of a neuronal functional amyloid implicated in memory persistence in Drosophila. Science. 367(6483):1230-1234. doi: 10.1126/science.aba3526 (2020)
- Nil Z, Hervas R, Gerbich T, Leal P, Yu Z, Saraf A, Sardiu M, Lange JJ, Yi K, Unruh J, Slaughter B, Si K. Amyloid-like assembly activates a phosphatase in the developing Drosophila embryo. Cell. 179(3):801. doi: 10.1016/j.cell.2019.09.033. (2019)
- Fernandez-Ramirez MDC, Hervas R, Galera-Prat A, Laurents DV, Carrion-Vazquez M. Efficient and simplified nanomechanical analysis of intrinsically disordered proteins. Nanoscale. 10(35):16857-16867. (2018)
- Hervas R*, Li L*, Majumdar A, Fernández-Ramírez MDC, Unruh JR, Slaughter BD, Galera-Prat A, Santana E, Suzuki M, Nagai Y, Bruix M, Casas-Tintó S, Menéndez M, Laurents DV, Si K, Carrión-Vázquez M. Molecular basis of Orb2 amyloidogenesis and Blockade of memory consolidation. PLOS Biol. 14(1):e1002361. doi: 10.1371/journal.pbio.1002361. (2016)
- Mompeán M, Hervas R, Xu Y, Tran TH, Guarnaccia C, Buratti E, Baralle F, Tong L, Carrión-Vázquez M, McDermott AE, Laurents DV. Structural evidence of amyloid fibril formation in the putative aggregation domain of TDP-43. J Phys Chem Lett. 6(13):2608-15. (2015)
- Hervas R*, Oroz J*, Galera-Prat A, Goñi O, Valbuena A, Vera AM, Gómez-Sicilia A, Losada-Urzáiz F, Uversky V, Menéndez M, Laurents DV, Bruix M, Carrión-Vázquez M. Common features at the start of the neurodegeneration cascade. PLOS Biol 10(5): e1001335. doi: 10.1371/journal.pbio.1001335 (2012)
- Oroz J, Hervas R and Carrión-Vázquez M. Unequivocal Single-Molecule Force Spectroscopy of Proteins by AFM using pFS Vectors. Biophys J 102:682-690 (2012)
- Valbuena A, Oroz J, Hervas R, Vera AM, Rodríguez D, Menéndez M, Sulkowska JI, Cieplak M and Carrión-Vázquez M. On the remarkable robustness of scaffoldins. Proc. Natl. Acad. Sci. USA 106(33):13791-6 (2009)
- Ferrer Foundation | Ph.D. Fellowship Severo Ochoa, 2008
- CEU-Canon Foundation | European-Japanese Fellowship, 2013
- Carrión-Vázquez M, Hervas R, Oroz J.
Expression vectors in the Nanomechanical Analysis of Proteins Application in Therapy and Diagnosis – CSIC-CIBERNED, 2010, P201031846, PCT/ES2011/070867 - Carrión-Vázquez M, Hervas R. Use of QBP1 Peptide for the Inhibition of Memory Consolidation - CSIC-CIBERNED, 2015. 15382176

