BSc, PhD (HKU)
Assistant Professor

L1-53, Laboratory Block,
21 Sassoon Road, Hong Kong
T +852 3917 9172
F +852 2817 0857
cyschung@hku.hk
Chemical biology: application of synthetic chemicals and chemistry techniques to study and manipulate biological systems
Understanding functions and activities of biomolecules at molecular level are crucial, as this can significantly increase our knowledge on physiology and pathology which can facilitate development of effective diagnosis and therapy for diseases. Yet, complex biological environments make studies on specific target or signaling event very challenging. Chemical compounds can be valuable tools for tackling this challenge, as generally they can be synthesized in large scale and high purity with good characterization, as compared to biological modalities. More importantly, by good design on their chemical structures, these compounds can be readily functionalized for serving specific purpose to study, image or profile target biomolecules, or even modulate activities of the targets and exhibit therapeutic effects.
Our lab has been working on chemical biology to develop: (1) chemoproteomics probes to identify new druggable hotspots in disease samples; (2) molecular probes for investigating cellular redox biology; (3) therapeutic covalent ligands for targeted therapy
(1) New cysteine-reactive probes to expand the pool of ligandable cysteines for biological studies and drug development
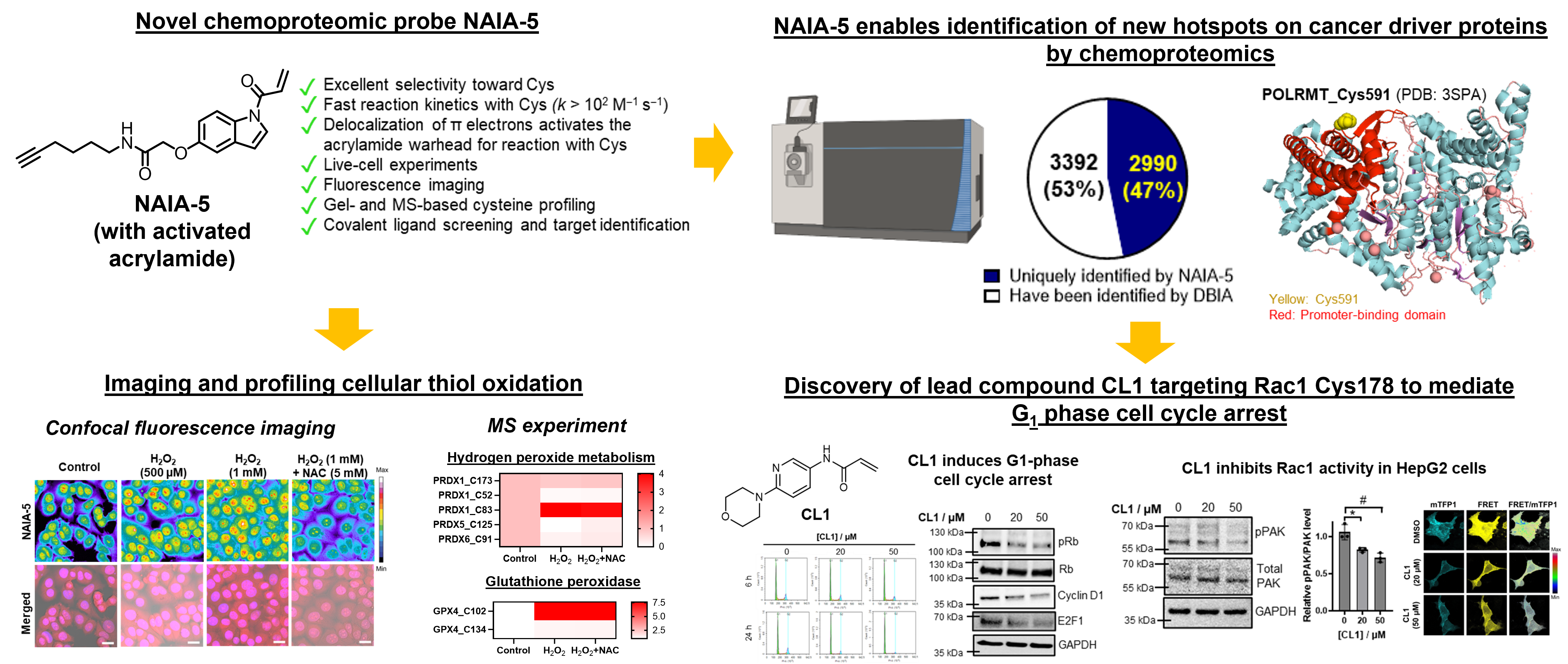
Understanding functions and reactivity of cysteines on proteins have arouse lots of interest. This is not only for getting better ideas on biological processes initiated/regulated by proteins containing these functional cysteines, but also for the huge potentials to develop drug compounds targeting these cysteines for therapy.
Activity-based protein profiling (ABPP) is one of the most widely used platforms for proteome-wide cysteine profiling to identify functional cysteines. Cysteine-reactive probe is the key component in ABPP platform and can define the pool of ligandable cysteines and hence the population of proteins that can be targeted by covalent ligands. The conventional cysteine-reactive probe, iodoacetamide-alkyne (IAA), shows only fair reaction kinetics and selectivity with cysteine. Together with its high cellular toxicity and low biostability, this limits the full potential of ABPP platform for biological studies and drug research.
Against this backdrop, our lab is developing novel cysteine-reactive probes which show better cysteine reaction kinetics and selectivity. Together with their high biostability and low cytotoxicity, they have been found to capture more cysteines than IAA in cell lysates and live cells, in both gel-based and fluorescence imaging experiments. More interestingly, in MS experiments, our probes capture a larger and a significantly different population of cysteines than IAA. This should expand the pool of ligandable hotspots in whole-proteome cysteine profiling experiments, and facilitates further research and study on the development of new covalent ligands and potential lead compounds for targeting these new proteins for therapy.
Representative work:
Koo, T.-Y.,† Lai, H.,† Nomura, D. K., & Chung, C. Y.-S.* (2023). N-Acryloylindole-alkyne (NAIA) enables imaging and profiling new ligandable cysteines and oxidized thiols by chemoproteomics. Nature Communications, 14(1), 3564. (†These authors contributed equally) https://doi.org/10.1038/s41467-023-39268-w
(2) New chemical tools and technologies to study cellular redox biology

Cellular redox biology is governed by interesting classes of redox-active molecules, which can mediate reduction/oxidation of biomolecules. Notably examples of redox-active molecules include reactive oxygen species (ROS) such as hydrogen peroxide (H2O2) and superoxide (O2-), and hydrogen sulfide (H2S) which belongs to reactive sulfur species (RSS) family. These redox-active molecules were once classified as detrimental compounds in biology and known to contribute to serious disease development such as cancer and neurodegenerative disorders. Yet, recently these redox-active molecules are found to be essential to life and can serve as important signaling molecules. This is mainly through their Redox Biology, which induces reduction/oxidation of biomolecules and subsequently changes their functions, activities and downstream signals.
Despites nowadays there are better ideas on redox signaling and its biological functions, the molecular mechanism on initiation, transduction and regulation of redox signaling, as well as the identity of proteins involved in the signaling process, remain insufficiently understood. This is because redox modifications of proteins are reversible, dynamic and unstable. In contrast to protein phosphorylation/dephosphorylation which are more stable modifications, there are no good conventional biochemical and biological experiments to study protein redox modifications and hence Cellular Redox Biology.
To overcome this challenge, our lab is developing new chemical probes that can: (1) induce “permanent covalent tag” onto proteins associated with Redox Biology. This allows us to identify the proteins by proteomics and mass spectrometry (MS)-based experiments. (2) We can also real-time visualize redox signaling events by turning the permanent covalent tag into a fluorescent one. This enables fluorescence imaging with superior spatial resolution.
Representative work:
Lai, H., & Chung, C. Y.-S.* (2024). Superoxide-responsive quinone methide precursors (QMP-SOs) to study superoxide biology by proximity labeling and chemoproteomics. RSC Chemical Biology, 5(9), 924–937. https://doi.org/10.1039/D4CB00111G
**In the themed collection: 2024 RSC Chemical Biology Emerging Investigators
(3) Therapeutic covalent ligands for cancer therapy
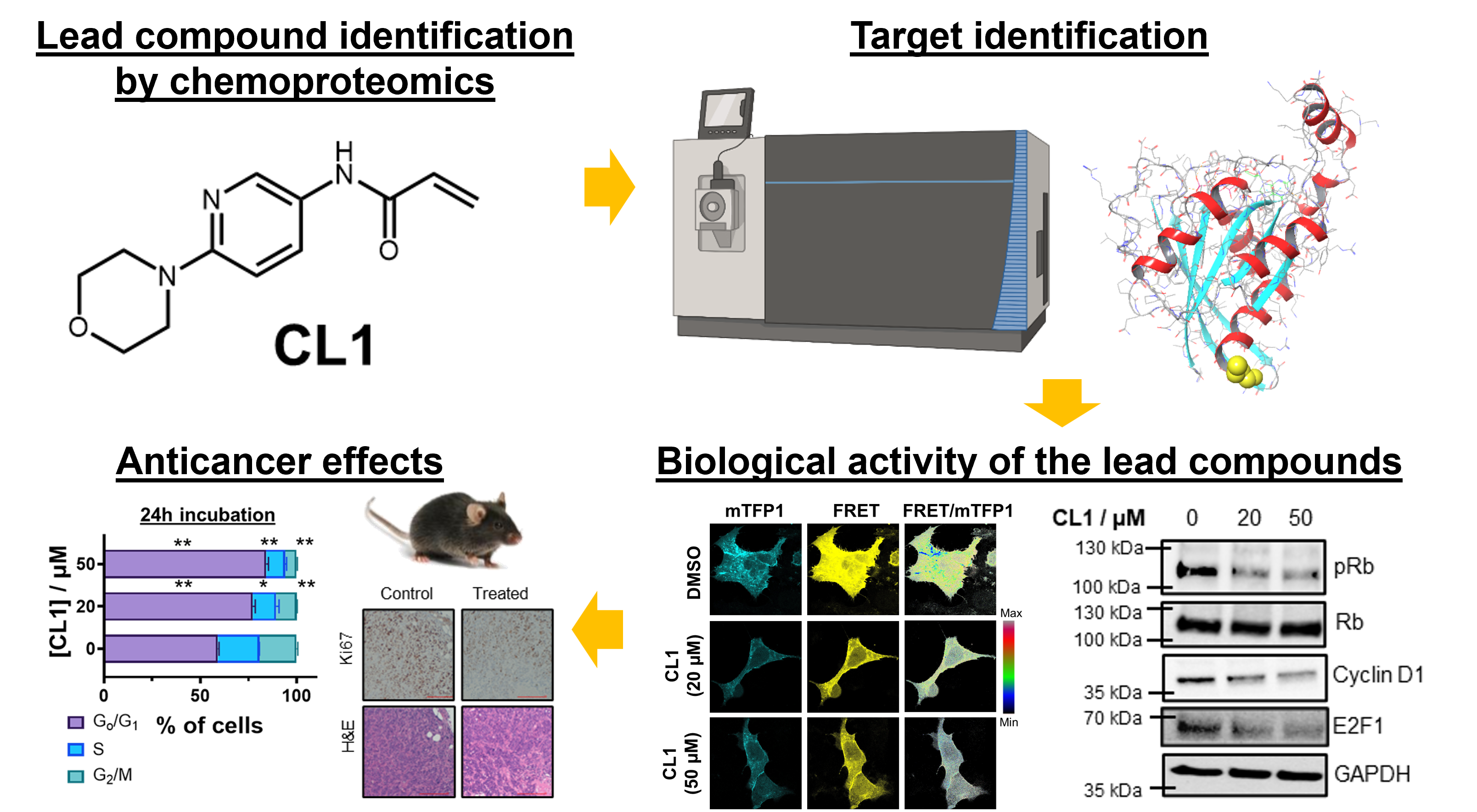
As compared to chemotherapy, targeted therapy is generally more specific and shows less toxic side effects, and is considered to be a better treatment option to cancer patients. Nonetheless, only a small population of cancer-related proteins have been drugged by targeted therapeutic agents, thus not much alternative treatment options are available when drug resistance develops or poor prognosis is found. This limitation can be explained by the fact that close to 90% of human proteins are lack of nice and deep pockets for drug compound binding, and they were once considered as undruggable.
Recently, covalent drugs have been successfully developed to target G12C mutation in KRAS, one of the most famous undruggable proteins. This motivates us to explore covalent ligands to target new cancer driver proteins and investigate their potential applications for targeted cancer therapy. Through chemoproteomics-enabled screening platform, we have identified novel covalent ligands targeting different cancer driver proteins which have not been drugged so far. By integrative chemical biology and MS-based ABPP experiments, we have confirmed the specific binding of the covalent ligands on the cancer driver proteins in cancer cells. Some of the covalent ligands have been found to show promising in vivo antitumor effects, demonstrating the good potential of using these compounds for targeted cancer therapy.
Example 1: Novel covalent ligand targeting RhoA Cys16 for colorectal cancer (CRC) treatment
RhoA is a key cancer driver and potential colorectal cancer (CRC) therapy target but remains undrugged clinically. Using activity-based protein profiling (ABPP) and mass spectrometry (MS), we identified CL16, a covalent inhibitor targeting the unique Cys16 on RhoA subfamily, which confers high specificity over other Rho family proteins. Cys16 is adjacent to the nucleotide-binding pocket and switch regions, which are critical for RhoA function. The binding by CL16 effectively disrupts GTP binding and inhibits RhoA activity in CRC cells, leading to cytotoxic killing of CRC cells through cell-cycle arrest and apoptosis. In mouse CRC models, CL16 exhibits strong antitumor and antimetastatic effects, promotes T cell infiltration into the tumor microenvironment, and shows no observable toxicity. Our findings suggest that covalent targeting of the druggable Cys16 on RhoA offers a promising strategy for CRC treatment, providing a foundation for developing specific RhoA inhibitors for clinical application.
Representative work:
Koo, T.-Y.,† Li, J. Y. K.,† Lee, N.-S.,† Chen, J., Yip, H. Y.-Y., Huang, I. B., Ng, K.-Y., Yan, H. H. N., Leung, S. Y., Ma, S., Zhou, J., & Chung, C. Y.-S.* Chemoproteomics-driven discovery of a covalent inhibitor targeting Cys16 on RhoA in colorectal cancer. Cell Chemical Biology, In press. https://doi.org/10.1016/j.chembiol.2025.08.004
Example 2: Novel covalent ligand targeting AGPAT4 Cys228 for hepatocellular carcinoma (HCC) treatment
The development of cancerous cells leads to considerable changes in metabolic processes to meet the demands of tumor growth. Tumor lineage plasticity has been identified as a key factor in therapy resistance and tumor recurrence. Herein, we showed one aspect of this plasticity to be abnormal glycerophospholipid metabolism, specifically the presence of a metabolic protein called 1-acylglycerol-3-phosphate o-acyltransferase 4 (AGPAT4). Through a chemical biology approach, a cysteine-reacting compound that specifically targets AGPAT4 at the Cys228 residue and therefore hinders its acyltransferase activity was identified and found to work synergistically with sorafenib in suppressing HCC in tumor xenograft models derived from patients with preclinical HCC and sorafenib-resistant HCC. Toxicological analysis revealed minimal side effects associated with the covalent inhibitor. In conclusion, the plasticity of tumor lineages induced by AGPAT4 represents a potential target for HCC treatment and could expand the effectiveness of sorafenib treatment, offering new possibilities for HCC therapy
Representative work:
Ng, K.-Y.,† Koo, T.-Y.,† Huang, I. B., Lee, T. K.-W., Fong T.-L., Gao, Y., Wong, T.-L., Gao, Y., Yun, J.-P., Guan, X.-Y., Liu, M., Chung, C. Y.-S.* & Ma, S.* AGPAT4 targeted covalent inhibitor potentiates targeted therapy to overcome cancer cell plasticity in hepatocellular carcinoma. Science Translational Medicine, 17(809), eadn9472. https://doi.org/10.1126/scitranslmed.adn9472
Chemical biology, chemoproteomics, therapeutic covalent ligands
Redox biology and molecular probes
Cancers
Faculty Outstanding Research Output Award, LKS Faculty of Medicine, HKU (2023)
2023
Croucher Postdoctoral Fellowship (2016-18)
2016-18
8. Superoxide-responsive quinone methide precursor (QMP-SO) and method of use (U.S. Patent Application No. 63/669,255)
7. N-Acryloylindoles and methods of use (U.S. Patent Application No. 63/365,391; Chinese Patent Application No. 202310593748.6).
6. Metal-Directed Acyl Imidazole Strategy (U.S. Patent Application No. 63/020,014).
5. mTORC1 inhibitors for activating autophagy (WO2020146779)
4. Probes for detection of copper (WO2020226972)
3. mTORC1 modulators (US10807951; US20190112268; WO2019075386)
2. Puromycin-based probes and methods of use thereof (US20210061842; WO2019183270)
1. Gold porphyrin-PEG conjugates and method of use (US20170157261; US11135310; CN108779124; EP3386986)
Last Update : 2025-12-10