Jun 19, 2023
Press Release: HKUMed establishes the first platform to slash time engineering and selecting the best precise genome editors for applications
Press Release (2023-06-19):
HKUMed establishes the first platform to slash time engineering and selecting the best precise genome editors for applications
A research team from the LKS Faculty of Medicine, the University of Hong Kong (HKUMed) has developed a new way to breakthrough the current limited throughput in optimising precise genome editors at scale, engineer hundreds (or more) of base editor variants in parallel instead of current laborious one-by-one testing, and informing users of the most suitable ones for therapeutic genome editing. The finding has been published in Cell Systems [link to publication] and a patent application has been filed based on this work.
Background
Base editing is a newer CRISPR-based genome editing technology and emerges to be a safer tool for tackling genetic diseases with single-base mutations (such as sickle cell disease, familial hypercholesterolemia, etc.) at the DNA by correcting them back to their normal form. However, existing base editing can result in different outcomes depending on the type and version of the base editor used, the sequence composition of the target DNA, and the position of the DNA base(s) to-be-converted. Picking a suboptimal base editor for application can generate incorrect edits and extra mutations around the target DNA base, which could cause undesired effects. Currently, laborious exhaustive one-by-one testing has to be done to characterise the editing performance of the tens of available base editors to optimise their use on each therapeutic locus. In addition, many therapeutic loci do not have an existing optimised base editor for precise editing yet. Despite the efforts worldwide, creating a new base editor can take months or years using conventional methods.
Research methods and findings
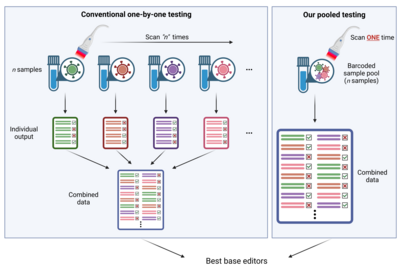
HKUMed’s research team successfully develops a platform coupling a base editor reporter system with CombiSEAL,[1]a state-of-the-art technology previously developed, to quickly engineer hundreds (or more) of base editor variants in parallel, with the combinations of varying enzymatic deaminase domain and CRISPR/ Cas9-based DNA-recognition domain. The compatibility and performance of these variants were yet to be characterised and compared in a head-to-head manner. Therefore, the team applied the platform to quantitatively readout each variant’s editing efficiency, purity, sequence motif preference, and bias in generating single and multiple base conversions in human cells, which helps select the most suitable ones for therapeutic target by generating a particular type of base conversion with maximal efficiency and minimal undesired edits.
The team extended the use of the platform to further enhance the efficiency of the current base editor system. The team members performed a screen focusing on engineering the stem-loop-2 region of the sgRNA (a single guide RNA) scaffold used in the base editor system, and successfully identified two novel sgRNA scaffold variants, SV48 and SV240, that outperform the wild-type scaffold to achieve greater (up to 2.2-fold higher) base editing efficiency.
Furthermore, the team demonstrated that the platform not only can be used for base editor characterisation and screening, but also is compatible with other precise genome editor systems such as prime editors. This could expand the scope of the work in search for other suitable editors to correct genetic mutations at therapeutic targets where base editor is not applicable.
Research significance
This platform is powerful in accelerating the engineering of next-generation precise genome editors and the adaptation of these editors in future therapeutic uses. ‘It is like an accelerated check-out process in stores. Since all product items (i.e. base editor variants) are tagged with a barcode, when it comes to the check-out counter barcode scanner, we need to only put all items in bulk into the basket at the check-out counter. The scanner can automatically identify all items and complete the payment (i.e. base editing performance analysis in our case). There is no need to individually test each base editor one-by-one.’ Dr Alan Wong Siu-lun, Associate Professor of the School of Biomedical Sciences, HKUMed, explained.
About the research team
The research was led by Dr Alan Wong Siu-lun, Associate Professor, School of Biomedical Sciences, HKUMed. John Fong Hoi-chun, PhD student, was first author, with assistance from Dr Chu Hoi-yee and Dr Zhou Peng, postdoctoral fellows, School of Biomedical Sciences, HKUMed.
Acknowledgement
This work was supported by the Hong Kong Research Grants Council (17102022 and 17101820), the National Natural Science Foundation of China Excellent Young Scientists Fund (32022089), and the Centre for Oncology and Immunology under the Health@InnoHK initiative funded by the Innovation and Technology Commission, The Government of HKSAR.
Media enquiries
[1] Choi G.C.G., et al. Combinatorial mutagenesis en masse optimizes the genome editing activities of SpCas9. Nature Methods 16, 722-730 (2019)

