Feb 02, 2023
Press Release: HKUMed discovers a new tumour suppressive gene which boosts personalised treatment response in breast cancer
Press Release (2023-02-02):
HKUMed discovers a new tumour suppressive gene which boosts personalised treatment response in breast cancer
Source: www.med.hku.hk/en/news/press/20230202-aktip-breast-cancer
A research team from the LKS Faculty of Medicine, the University of Hong Kong (HKUMed) discovered that somatic deletion of a tumour suppressor gene AKTIP promotes luminal breast cancer development and resistance to endocrine therapy. The findings are now published in Cell Reports [link to publication].
Background
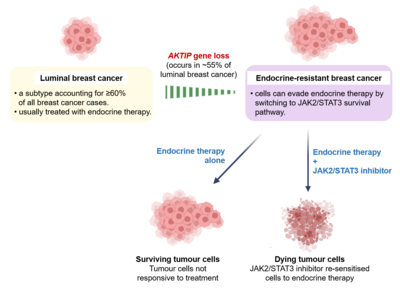
Breast cancer is the most common cancer and the third leading cause of cancer death among women in Hong Kong [1], and which can be classified into several molecular subtypes. Each subtype has distinct clinical characteristics, genetic profiles and treatment guidelines. While the disease can be hereditary with inherited mutations in genes such as BRCA1, the majority of the breast cancer cases are somatic resulted from non-inherited mutations that are acquired during one’s lifetime. The luminal breast cancer, which expresses a hormone receptor called estrogen receptor α (ERα), is the most common subtype and constitutes 60-70% of all breast cancer cases. Because the cancer cells express ERα which fuels cancer development, targeting ERα by therapeutic agents (endocrine therapy) is the cornerstone of management for luminal breast cancer. However, one third of luminal breast cancer patients who initially respond to endocrine therapy eventually develop resistance to the therapy. Deletion of the AKTIP gene is observed in about 55% of luminal breast cancer cases. Despite the high occurrence, the consequence of the gene deletion was unknown.
Research methods and findings
Through multi-omics and molecular biology approaches using breast cancer cell lines, clinical samples, mouse model and cancer patient-derived organoids, the team made an intriguing discovery that loss of the AKTIP gene promotes breast cancer through increasing the protein expression level of ERα. Consistent with the pro-cancer consequences observed in these experimental models, an analysis of patient data showed that luminal breast cancer patients with AKTIP gene deletion has worse survival. Importantly, breast cancer cells with AKTIP loss are resistant to endocrine therapy. This endocrine resistance is due to a concurrent activation of another survival pathway JAK2/STAT3, which represents an alternative escape pathway utilised by the cancer cells when ERα is inhibited. Building on this finding, the team further found that blocking this alternative escape pathway by the addition of JAK2/STAT3 inhibitor can overcome the resistance.
Research significance
This study identified a new driver aberration of luminal breast cancer and the therapeutic possibilities targeting breast tumours with AKTIP gene deletion. ‘We present clear evidence that deletion of AKTIP is a prognostically and therapeutically relevant chromosomal mutation in luminal breast cancer. Our findings that JAK2/STAT3 inhibitor can reverse the endocrine resistance caused by AKTIP deletion need to be further investigated. Genomic data from tumour DNA profiling are increasingly guiding cancer care in which tailored therapy can be formulated based on the gene status of the cancer patient. This precision medicine approach can kill tumour cells efficiently with less toxic side effects. The incorporation of AKTIP gene status as predictive biomarker may refine the treatment strategy for luminal breast cancer,’ explained by Dr Lydia Cheung Wai-ting, Assistant Professor of School of Biomedical Sciences, HKUMed.
About the research team
The research was led by Dr Lydia Cheung Wai-ting, Assistant Professor, School of Biomedical Sciences, HKUMed. Team members included Angel Ng Sau-ni, Dr Zhang Shibo, Dr Victor Mak Chun-yin , Zhou Yuan and Yuen Yin , School of Biomedical Sciences, HKUMed. Other researchers contributing to the research included Dr Sharma Rakesh, Centre for PanorOmic Sciences, HKUMed; Professor Lu Yiling, University of Texas MD Anderson Cancer Center, the United States; Dr Zhao Wei, National Cancer Institute of National Institutes of Health, the United States; Professor Zhuang Guanglei, School of Medicine, Shanghai Jiao Tong University; and Dr Pang Herbert, School of Public Health, HKUMed.
Acknowledgement
The work was supported by funding from the National Natural Science Foundation of China Excellent Young Scientists Fund (Hong Kong and Macau) (Grant No. 82022078).
Media enquiries
[1] Hong Kong Cancer Registry, Hospital Authority. https://www3.ha.org.hk/cancereg/topten.html

