Jun 02, 2022
Press Release: HKUMed-QMUL research team identify biomechanical and molecular signals that trigger early progression of atherosclerosis
Press Release (2022-06-02):
HKUMed-QMUL research team identify biomechanical and molecular signals that trigger early progression of atherosclerosis
Source: https://www.med.hku.hk/en/news/press/20220602-early-progression-of-atherosclerosis
A joint research team at LKS Faculty of Medicine, The University of Hong Kong (HKUMed) and Queen Mary University of London (QMUL) has discovered the molecular mechanisms of how pathological condition-mimicking mechanical stimuli lead to the early progression of atherosclerosis, a leading cause of vascular disease worldwide. The research findings provide new insights into the mechanosensitive pathway of vascular smooth muscle cell during the initial phase of atherosclerosis and have been recently published in Science Advances (Link to publication).
Background
Major risk factors of atherosclerosis include ageing-associated changes of the mechanical properties of the arterial wall and hypertension. The phenotypic switching, proliferation, and apoptosis of vascular smooth muscle cell result in the intimal thickening and mark the first stage of atherosclerosis. However, the molecular mechanisms of how vascular stiffness and blood pressure changes promote the phenotypic switch of vascular smooth muscle cell remain largely unknown.
Cofilin as the key regulator during the early stage of atherosclerosis
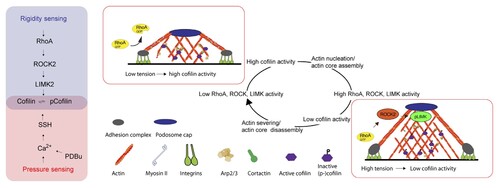
Applying the proteomic and microscopy approach, the HKUMed-QMUL research team found that the combination of cyclic hypertensive pressure stimulation and matrix compliance in extracellular microenvironment is necessary to give rise to a full phenotypic switch of vascular smooth muscle cell, including significant changes in atherosclerosis-associated protein expression, cytoskeletal organisation, and the formation of podosome. The phenotypic switching mediated by mechanical stimuli are confirmed in rat, mouse, bovine and human vascular smooth muscle cells.
The underlying principle of mechanosensitive response is orchestrated by cofilin, a cytoskeletal protein that regulates actin nucleation. The dephosphorylation status, rather than the expression, is known to be the key factor to regulate cofilin activities. Here, the HKUMed-QMUL research team identified the mechano-biological pathways that modulate cofilin phosphorylation in vascular smooth muscle cells. Specifically, pressure stimuli initiate the calcium-dependent slingshot activation that dephosphorylates cofilin, while the reduction of substratum stiffness suppresses the signal cascade of RhoA-ROCK2-LIMK2 and restrains cofilin phosphorylation. Collectively, higher pressure and softer substratum cause the decrease of cofilin phosphorylation, promote actin nucleation, and accelerate in the oscillatory nature of podosome. The formation of fast-oscillatory podosome, the signature event of the phenotypic switching of vascular smooth muscle cells, elevates the matrix degradation and vascular microenvironment disorganisation, and results in the early progression of atherosclerosis.
Research significance
‘Our findings highlight the role of extracellular microenvironment and underlying pressure and rigidity sensing mechanisms involved in atherosclerosis progression,’ said Dr Yu Cheng-han, Assistant Professor, School of Biomedical Sciences, HKUMed, who is one of the leading researchers of the study. ‘While regular exercise and balanced diet can effectively prevent or slow the progression of atherosclerosis, cofilin could be a potential target for mitigating atherosclerosis at early phase, paving the way for new therapeutic opportunities.’
About the research team
The study was conducted by Dr Yu Cheng-han, Assistant Professor at the School of Biomedical Sciences, HKUMed, his research postgraduate student Mr Brian Sit Hon-man, Miss Feng Zhen, and his collaborator Dr Thomas Iskratsch at QMUL. Dr Yu and his team also actively work on cutting-edge research projects in GTPase regulation, PI3K activation, and mechano-sensitive signalling.
Acknowledgement
This study was supported by the Research Grant Council - General Research Fund (#17122019).

